It is November 2020, and COVID-19 hasn’t disappeared, contrary to what particular figures of authority declared months ago. Despite the growing pandemic, I think one positive outcome of this year has been the gradual, widespread accessibility of testing to millions of Americans and the relentless pursuit of a vaccine for this highly contagious enemy.
After being exposed to a few people who were in contact with a person who was actively fighting the coronavirus, I decided to get tested. I knew that some pharmacies within the chain were offering COVID testing at specific locations. I booked an appointment within minutes from the pharmacy chain’s website. I was able to choose a time and location that was convenient for me. Unfortunately, all of the same-day rapid-testing appointments were booked so I had to book an appointment for a test that would not provide me with the results right away. I pulled up to the drive-thru testing site of the pharmacy, swirled a nasal swab up into both of my nostrils for 15 seconds each and dropped the swab into a test tube. That was it! I received my results 3 days by email following my appointment. The testing in and of itself was easy to follow and very brief, which is especially convenient for someone who doesn’t have much time to wait or who can sacrifice an entire day to get tested.
Undoubtedly, we know that the coronavirus is very real and very contagious, but how do we know when we might have it?
The answer is through testing! There are several types of tests available for the diagnosis of COVID-19 as well as tests to see if you have previously had COVID-19. According to the FDA, there are two approved types—diagnostic tests and antibody tests.1
What’s the difference between the two types of tests?
Diagnostic testing reveals if an individual has an active COVID-19 infection and can further be broken down into molecular tests and antigen tests. Molecular tests look for the presence of genetic material of the SARS-CoV-2 virus, the pathogen responsible for the COVID-19 infection. An antigen test looks for the presence of specific proteins on the surface of the SARS-CoV-2 virus. Both molecular and antigen tests can be used to detect the presence of a current infection. A positive antibody test reveals that the individual getting tested had a prior COVID-19 infection. Antibody tests look for the presence of antibodies developed by the individual’s immune system in order to fight off the SARS-CoV-2 virus. Both molecular and antigen tests are conducted via a nasal or nasopharyngeal (back of the nose and throat) swab. An antibody test involves blood work such as a finger stick or blood draw.1 Results for these tests can typically be expected within the same day with the exception of antigen tests which could see rapid results within 15-30 minutes.
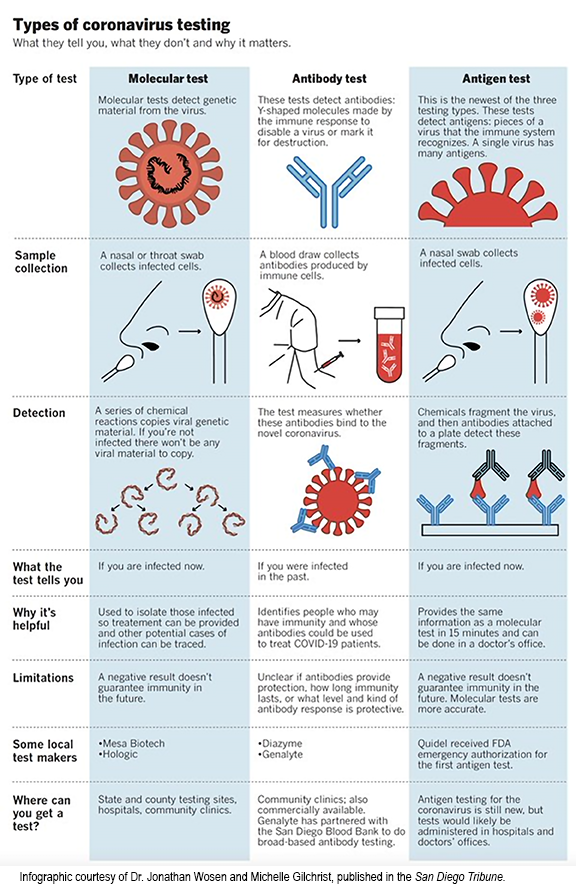
While there are multiple options when it comes to testing for the coronavirus, there are limitations to antigen and antibody testing. According to an article published in the respiratory medicine journal, The Lancet, the author states that “the results [of an antibody test] cannot tell you whether you are currently infected with SARS-CoV-2, nor whether you can infect others.” Additionally, if the test is conducted too soon after contracting the infection, there might not be antibodies yet developed to be detected.2 For antigen tests, they have a much lower sensitivity than PCR tests; therefore, it’s more likely to miss detecting an active COVID-19 infection.1 Albeit these limiting aspects of the only current FDA-approved tests, it’s imperative to remember that there exists limitations to everything, but don’t let these factors discourage you from getting tested when you display symptoms or feel unwell. Both methods of diagnostic testing allows individuals who test positive to receive care earlier in their infection state and be on their way to wellness. Additionally, contacts can be traced and the spread of the virus can be as limited as possible. Together, we can put an end to this seemingly endless nightmare and create a new normal in which we all thrive. For COVID-19 testing sites in the Philadelphia area, click here.
References
1. U.S. Food & Drug Administration [Internet]. Atlanta (GA): U.S. Food & Drug Administration; [updated 2020 Nov 6; cited 2020 Nov 10]. Available from: https://www.fda.gov/consumers/consumer-updates/coronavirus-disease-2019-testing-basics.
2. Burki TK. Testing for COVID-19. Lancet Respir Med. 2020 July;8(7): 63-64. https://www-sciencedirect-com.db.usciences.edu/science/article/pii/S2213260020302472?via%3Dihub
Written by:Nicole Lin
Doctor of Pharmacy Candidate 2023
Philadelphia College of Pharmacy
